Rapid natural tear production1
Rapid natural tear production1

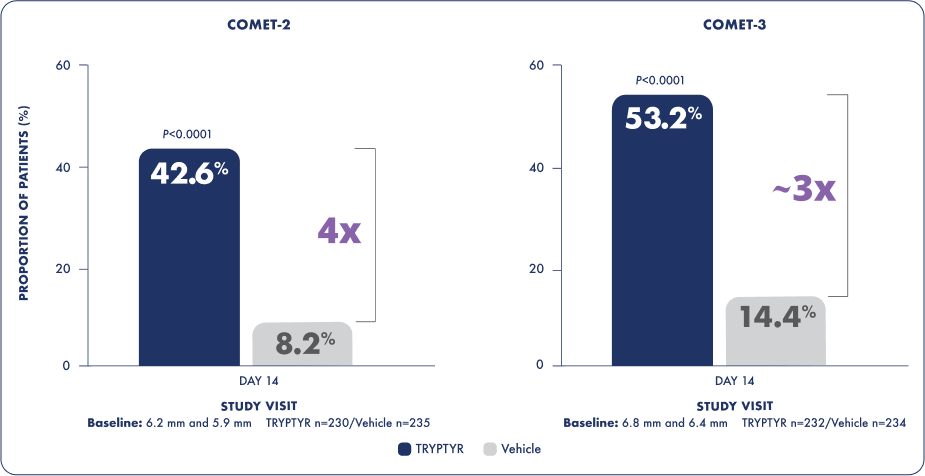
Up to 4x more patients achieved at least a 10 mm increase in natural tear production1*

TRYPTYR delivered natural tear production as early as Day 11


Symptom reduction on Day 282†
*42.6% and 53.2% with TRYPTYR versus 8.2% and 14.4% with vehicle on Day 14.
†In COMET-2 only. COMET-3 did not meet statistical significance.
UP TO 4x MORE PATIENTS Achieved AT LEAST A 10 MM INCREASE IN NATURAL TEAR PRODUCTION1*
Primary endpoint:
Proportion of patients achieving ≥10 mm increase from
baseline in unanesthetized Schirmer score on Day 14
*Versus vehicle.
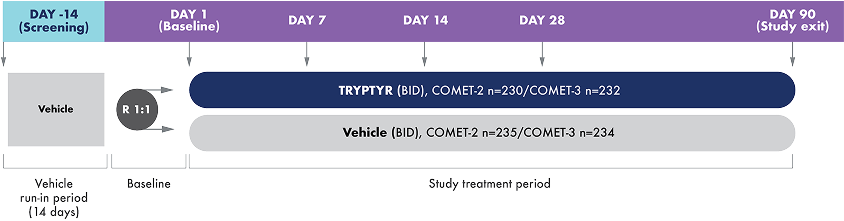
COMET-2 and COMET-3 were identical Phase 3, randomized, multicenter, double-masked, vehicle-controlled studies to investigate the efficacy and safety of TRYPTYR for dry eye disease.
TRYPTYR or vehicle were administered 1 drop, twice daily for 90 days.
- Mean age: 61 years (range, 30-93 years)
- 74.8% of patients were female
PRIMARY EFFICACY ENDPOINT
Proportion of patients who achieved a ≥10 mm increase from baseline in unanesthetized Schirmer score on Day 14*
FIRST (KEY) SECONDARY ENDPOINT
Change from baseline in global SANDE score on Day 28†
OTHER SECONDARY ENDPOINTS
Proportion of patients who achieved a ≥10 mm increase from baseline in unanesthetized Schirmer score on Day 1 and Day 90*
Change from baseline in unanesthetized Schirmer score on Day 1, Day 14, and Day 90*
*At each study visit, unanesthetized Schirmer score was measured 3 minutes post-drop.
†SANDE, the Symptom Assessment iN Dry Eye questionnaire, quantifies both the frequency and severity of dry eye symptoms (dryness or ocular discomfort).
TRYPTYR DELIVERED NATURAL TEAR PRODUCTION as early as DAY 11,2
Other secondary endpoint:
Proportion of patients achieving ≥10 mm increase from baseline in unanesthetized Schirmer score on Day 1 and Day 90
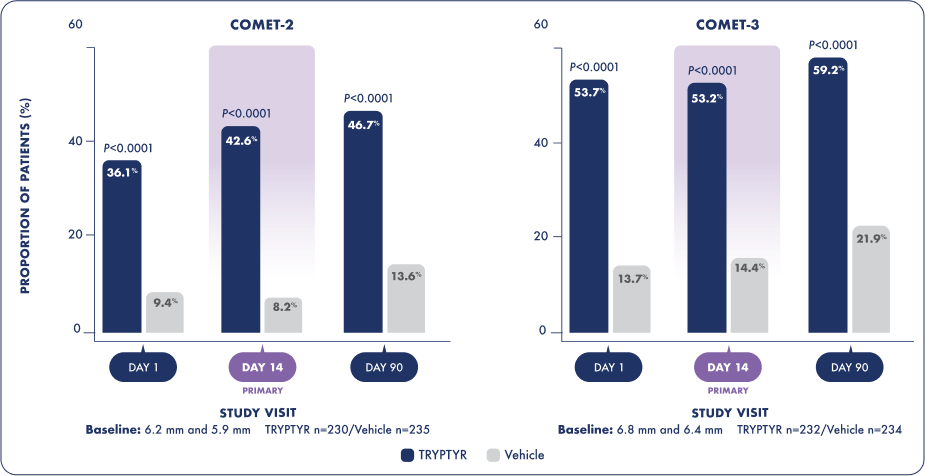
Consistent results were observed at all timepoints through Day 901
TRYPTYR SYMPTOM REDUCTION ON DAY 282*
First (key) secondary endpoint:
Change from baseline in global SANDE score by visit
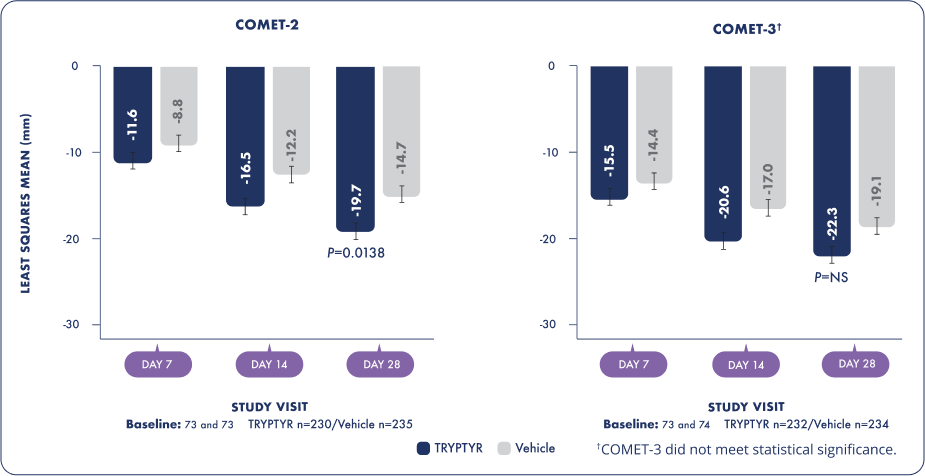
Frequency and severity of dry eye symptoms were measured using global SANDE score
*Versus vehicle.
SAFETY AND TOLERABILITY:
4 clinical trials with 766 patients treated with TRYPTYR1,2
No serious adverse reactions related to TRYPTYR (n=766)
THE MOST COMMON OCULAR ADVERSE REACTION WAS INSTILLATION SITE PAIN* REPORTED IN 50% OF PATIENTS

AN AVERAGE OF 86% OF PATIENTS EXPERIENCING PAIN SENSATION REPORTED SENSATION LASTING 1 MINUTE OR LESS

*Instillation site pain was defined as burning or stinging.
Get started with TRYPTYR
REFERENCES
- TRYPTYR. Prescribing Information. Alcon, Inc., 2025.
Data on File. Alcon, Inc. Fort Worth, TX.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
Potential for Eye Injury and Contamination: To avoid the potential for eye injury and contamination, advise patients not to touch the vial tip to the eye or other surfaces.
INDICATIONS AND USAGE
TRYPTYR (acoltremon ophthalmic solution) 0.003% is indicated for the treatment of the signs and symptoms of dry eye disease (DED).
Use with Contact Lenses: TRYPTYR should not be administered while wearing contact lenses. If contact lenses are worn, they should be removed prior to administration of the solution. Lenses may be reinserted 15 minutes following administration.
Adverse Reactions
In clinical trials, the most common adverse reaction was instillation site pain (50%).
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for the TRYPTYR Full Prescribing Information.